Sir William Robert Grove was a Welsh physical scientist best known for his pioneering role in the development of the fuel cell. Thus dubbed by many “the Father of the Fuel Cell”, Grove also made significant contributions to the establishment of the law of conservation of energy. On his birthday, let’s explore his innovations and legacy.
Following Unexpected Interests
Despite studying classics at Brasenose College at Oxford University, graduating in 1832, and going on to become a lawyer, joining the bar in 1835, Grove switched gears that same year to pursue his passion for science. He became a member of the Royal Institution of Great Britain, an organization for scientific education and research. Grove was interested in the electric cell, and he began experimenting with different electrodes and electrolytes. By 1838, he published his first scientific paper on this topic, “On a New Voltaic Combination”, which detailed an early design for a device that would later be called the Grove cell.

Sir William Grove circa 1877. This work is in the public domain.
The Grove Cell and the First Fuel Cell
In 1839, Grove developed a cell consisting of a zinc anode in dilute sulfuric acid and a platinum cathode in concentrated nitric acid. The design also featured a porous ceramic pot that separated the two substances and allowed ions to be exchanged. This became known as the Grove cell and, by some estimations, was a superior design to the contemporary Daniell cell due to its higher voltage and current.
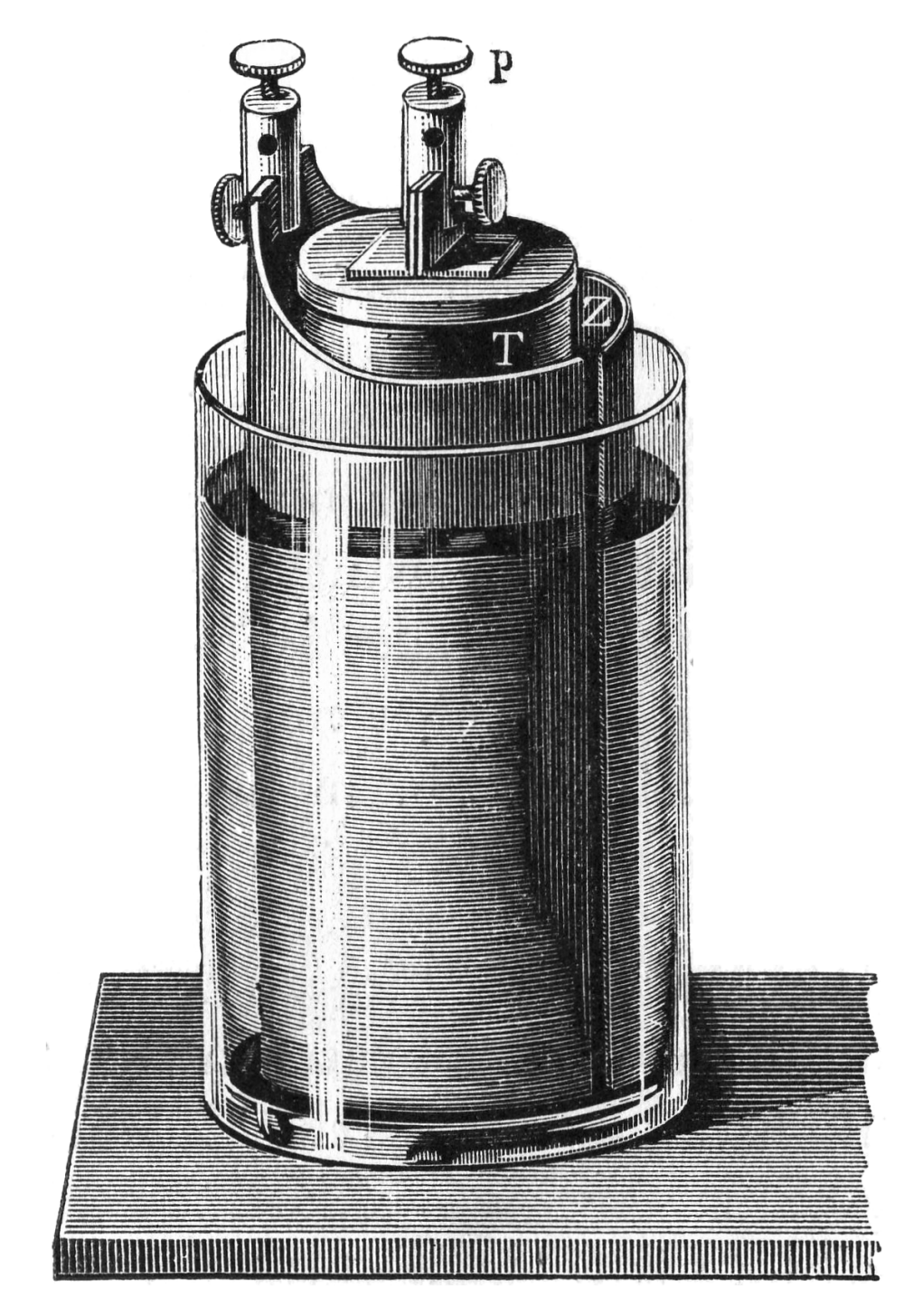
A Grove cell diagram from 1897. This work is in the public domain.
Unfortunately, the Grove cell design had some significant drawbacks: Platinum was prohibitively expensive, the cell emitted a poisonous gas (nitrogen dioxide), and it depleted quickly. German chemist Robert Bunsen developed an improved iteration called the Grove–Bunsen cell, which provided even greater current and voltage advantages over the Daniell cell and replaced the expensive platinum with carbon (in the form of coal and coke) — but still emitted the toxic gas. Despite the drawbacks of the Grove cell and its variants, the technology’s advent helped launch Grove’s scientific career. In 1840, he was elected a Fellow of the Royal Society of London and was appointed professor of experimental philosophy at the Royal Institution a year later.
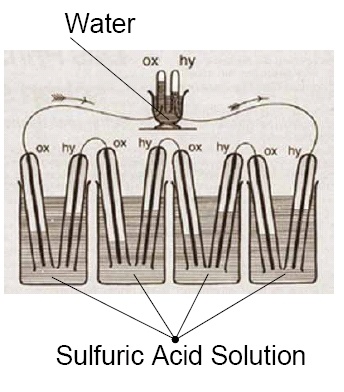
A diagram of the Grove cell. This is a work of the U.S. federal government and is, therefore, in the public domain.
The First Fuel Cell
Grove continued his experimentation with electric cells by using gases rather than liquids as electrolytes. The result of this work was what he called a “gas voltaic battery” and what today is considered the first fuel cell, developed in 1842. This gas battery decomposed steam into hydrogen and oxygen gas. Grove wrote in detail on the electrical current created as a result of the chemical activity inside the battery and the ability of the produced current to separate water into its parts. In this process, he saw a connection between forces of nature such as electricity and chemical energy.
With this battery, Grove became the first person to demonstrate the thermal dissociation of molecules into their constituent atoms, and some recognizable scientific figures were invited to his first demonstration. This list included Michael Faraday (the English chemist and physicist), John Peter Gassiot (the amateur scientist), and Edward William Brayley (a notable science author).
On the Correlation of Physical Forces
Grove’s “gas voltaic battery” laid the groundwork for his seminal work, “On the Correlation of Physical Forces”, published in 1846. He observed that when gases were combined to form a liquid, they released enough energy to decompose the liquid into a gas. This cemented a correlation for Grove, who would go on to publish works and speak on how the forces of nature could be converted into one another, how they could neither be created nor destroyed, and how they were ultimately just one force. In “On the Correlation of Physical Forces”, he argued that “heat, light, electricity, magnetism, chemical affinity, and motion are all correlative, or have a reciprocal dependence,” and that “either may, as a force, produce or be convertible into the other; thus heat may mediately or immediately produce electricity, electricity may produce heat; and so of the rest.”
Grove’s correlation of natural forces was an important step in the development of our understanding of the conservation of energy, though it would be Hermann von Helmholtz in 1847, building on earlier work by James Prescott Joule, William Thomson, and Grove, who would qualitatively prove this theory.
Further Reading
Want to learn more about fuel cells and how simulation is powering industry innovation in the modern world? Check out the following blog posts:
- Building an app to optimize solid oxide fuel cells
- Modeling a two-phase nonisothermal zero-gap alkaline water electrolyzer
- How to cope with shunt currents when modeling an alkaline electrolyzer stack



Comments (0)