Current Distribution in a Chlor–Alkali Membrane Cell
Application ID: 1813
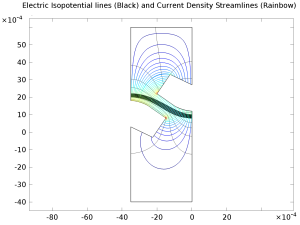
The chlor-alkali membrane process is one of the largest in industrial electrolysis with the production of roughly 40 million metric tons of both chlorine and caustic soda per year. Chlorine is used predominantly for the production of vinyl chloride monomer, which in turn is used for the production of poly vinyl chloride (PVC). Current density in membrane-cell technology has increased dramatically during the last decade as the membranes themselves have improved. This results in lower investment costs for greater production. However, the increase in current density implies an increase in power consumption if nothing is done to dampen the voltage increase. Advances in cell design including increased internal convection, decreased ohmic losses, and better membranes have allowed for large increases in current density with small increases in cell voltage. This example describes the current-density distribution in realistic anode and cathode structures in a membrane cell.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
- COMSOL Multiphysics® and
- either the Battery Design Module, Corrosion Module, Electrochemistry Module, Electrodeposition Module, or Fuel Cell & Electrolyzer Module
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Tabella delle Funzionalità and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



