Adsorption–Desorption Voltammetry
Application ID: 101531
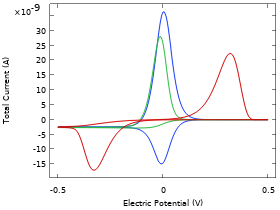
For an electrochemical reaction to occur, the reacting species usually needs to adsorb to the electrode surface before undergoing reduction or oxidation, after which the resulting product species desorbs back into the electrolyte.
If the rate of adsorption or desorption is slow in comparison to the electrochemical charge transfer step, the adsorption-desorption phenomena may have to be accounted for in a model.
This example investigates the impact of various kinetic parameters for adsorption, desorption and electron transfer when performing cyclic voltammetry on a planar electrode.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
- COMSOL Multiphysics® and
- either the Battery Design Module, Corrosion Module, Electrochemistry Module, Electrodeposition Module, or Fuel Cell & Electrolyzer Module
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Tabella delle Funzionalità and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.
