Model of Combustion Synthesis of Thermoelectric Calcium Cobaltates
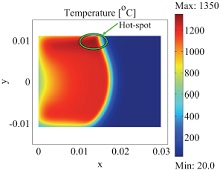
Self-propagating High-temperature Synthesis (SHS), a very economical synthesis of oxides was used in our lab to produce oxide materials. SHS process uses a highly exothermic reaction to convert reactants rapidly to pure products with minimal external energy input. This reaction is initiated by an igniter and reaction front propagates from the ignition through the rest of the sample. The fast heating up and cooling down steps in the reaction make it difficult to control the synthesis. A mathematical model is needed to study and optimize the synthesis conditions. In this work, we have generated a two-dimensional model of SHS reaction of calcium cobaltates using COMSOL Multiphysics. Momentum, heat, and mass transfers as well as reaction kinetics are included and coupled in our model. The model shows detailed reaction front movement and a narrow reaction zone typical for SHS reactions. Calculated results including the reaction rate and temperature profile are compared to experimental data to confirm its validity.
Download
- selig_presentation.pdf - 0.83MB
- selig_paper.pdf - 0.93MB



