La Galleria delle Applicazioni raccoglie un'ampia varietà di tutorial e di app dimostrative realizzati con COMSOL Multiphysics in diversi ambiti applicativi, inclusi quelli elettrico, meccanico, fluidico e chimico. E' possibile scaricare i file dei modelli e delle app demo pronti all'uso e le istruzioni step-by-step per costruirli, e utilizzarli come punto di partenza per le proprie simulazioni.
Lo strumento di Ricerca Rapida permette di trovare i modelli che si riferiscono alla propria area di interesse.
Si noti che molti degli esempi qui presentati sono accessibili anche tramite le Librerie delle Applicazioni incorporate nel software COMSOL Multiphysics® e disponibili dal menu File.
Steel pipelines are often subjected to complex stress/strain conditions in the oil and gas industry. Additionally, pipes are subjected to significant longitudinal strain due to soil movement. For the elastoplastic stress simulation, the Solid Mechanics interface is used with the small ... Per saperne di più
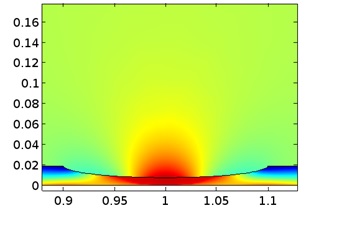
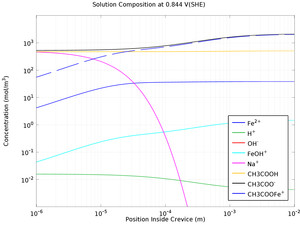
Mass transport limitations within thin crevices can often result in the local electrochemistry to differ significantly between the crevice opening (mouth) and end (tip), and as a result of the differences in local chemistry, corrosion may occur. This example models crevice corrosion of ... Per saperne di più
This example models galvanic corrosion between two different phases in a magnesium alloy for a representative cross-sectional microstructure configuration. The Phase Field interface is used here to model dissolution of a constituent phase leading to topological changes. The electrode ... Per saperne di più
Voltammetry is modeled at a microelectrode of 10um radius. In this common analytical electrochemistry technique, the potential at a working electrode is swept up and down and the current is recorded. The current-voltage waveform ("voltammogram") gives information about the reactivity and ... Per saperne di più
This tutorial example models the currents and the concentration of dissolved metal ions in a battery (corrosion cell) made from an orange and two metal nails. This type of battery is commonly used in chemistry lessons. Instead of an orange, lemons or potatoes can also be used. Per saperne di più
Steel structures immersed in seawater can be protected from corrosion through cathodic protection. This protection can be achieved by an impressed external current or by using sacrificial anodes. The use of sacrificial anodes is often preferred due to its simplicity. This example models ... Per saperne di più
This model simulates atmospheric galvanic corrosion of a busbar, which includes a copper flange, an aluminum alloy flange in contact with a zinc nut and bolt. The Secondary Current Distribution interface is used to solve for the electric potential in electrode domain and Current ... Per saperne di più
This 2D model demonstrates how to model a galvanic couple in which the corrosion of the anode causes a geometry deformation. The parameter data used is for an Magnesium Alloy (AE44) - mild steel couple in brine solution (salt water). Per saperne di più
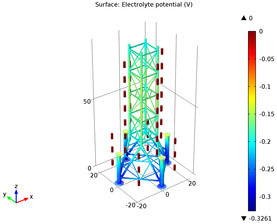
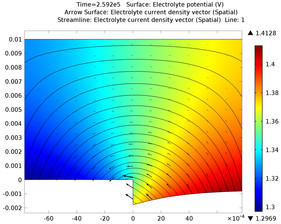
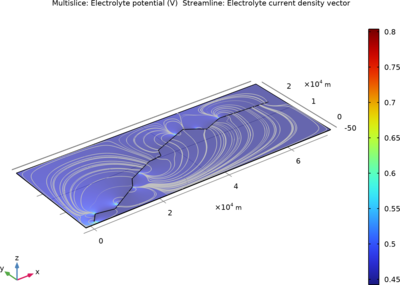
In this example, three parallel pipelines of length 68 km and a horizontal separation distance of 10 m between them are protected against corrosion by an impressed current cathodic protection (ICCP) system using a series of anodes. Each anode is connected to all three pipelines, ... Per saperne di più
Oxide jacking is the process by which reinforced concrete cracks, due to the corrosion of the reinforcing rebar rods. The corrosion process causes growth of an oxide layer on the rebar, which in turn causes internal stresses in the concrete. If the corrosion process is allowed to ... Per saperne di più