Introduction: Theory, Equations, and Interfaces
In Part 1 of this course on corrosion modeling using COMSOL Multiphysics® and the Corrosion Module, we introduce the two main branches of modeling corrosion: the corrosion process and corrosion protection systems. The corrosion process focuses on galvanic corrosion mechanisms such as pitting, crevice, atmospheric, and stress-induced corrosion. Corrosion protection systems involve cathodic protection (impressed current or sacrificial anodes), anodic protection, and internal pipeline protection. Both branches share the same governing equations including mass, charge and current conservation, and electrochemical reactions occurring at electrolyte–metal interfaces.
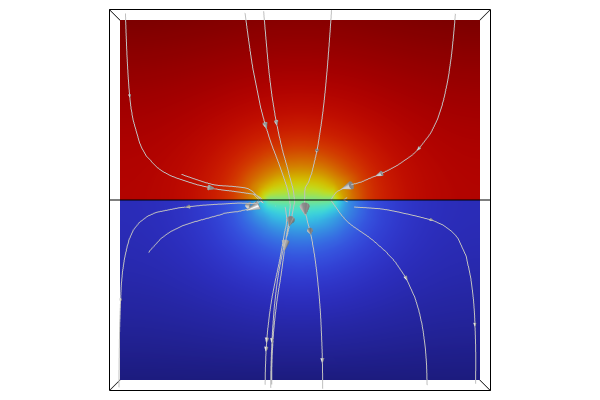
The Corrosion Module includes a variety of physics interfaces that can be used to model corrosion applications, such as primary, secondary, and tertiary current distribution and their associated losses, which can be coupled with fluid flow, heat transfer, and other physics depending on the model. Current distribution, along with Faraday’s law, is an important factor in analyzing corrosion, electrodeposition, and electrochemistry.
We also cover important conventions to keep in mind while modeling corrosion, such as current direction, overpotential, and electrochemical stoichiometry, as well as the major factors that affect the size and computation time of your simulation.
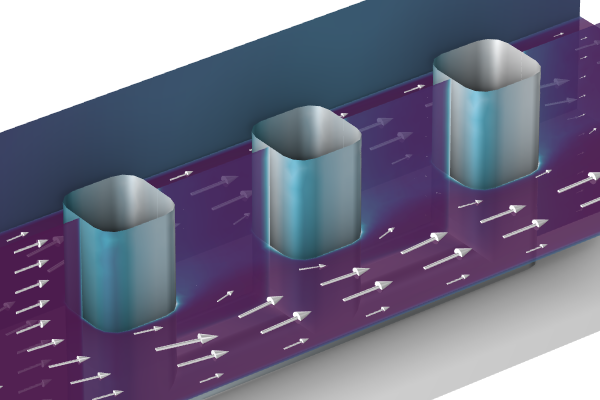
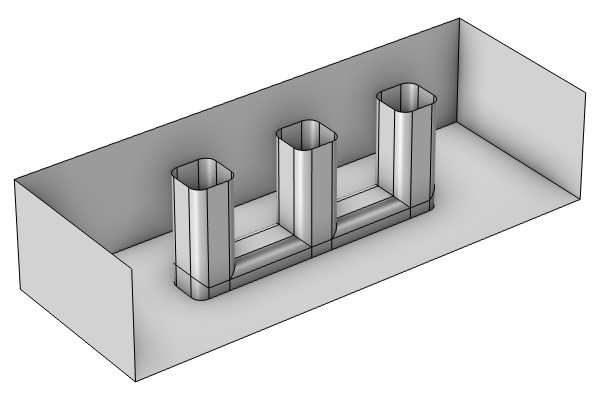
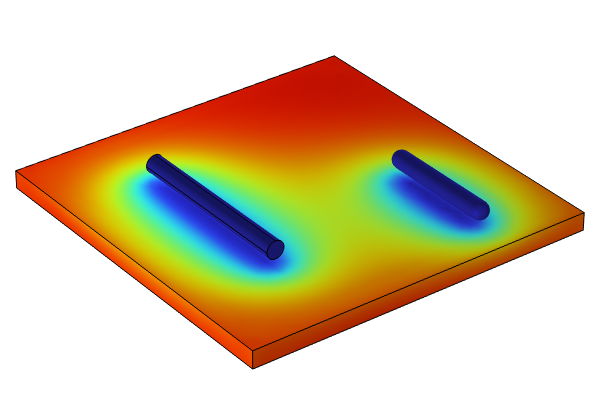
The Wire Electrode tutorial model is used here to demonstrate how to build a corrosion model step by step: importing the geometry; coupling multiphysics with laminar flow and species transport; and studying the primary, secondary, and tertiary current density distributions of an electrochemical cell.
Submit feedback about this page or contact support here.