Mitigating Moisture Migration in Pharma: A Coupled COMSOL and Experimental Approach
Understanding and controlling moisture migration is paramount in the pharmaceutical industry to ensure the quality, safety, and efficacy of drug products across their entire lifecycle, from raw material receipt to final product distribution. Uncontrolled moisture migration can lead to significant issues, including flowability problems, chemical degradation, undesirable physical changes, and compromised drug efficacy. This research investigates the complex interplay of moisture transport and material interactions within pharmaceutical powders under varying environmental conditions, leveraging a synergistic approach combining computational modeling with experimental validation.
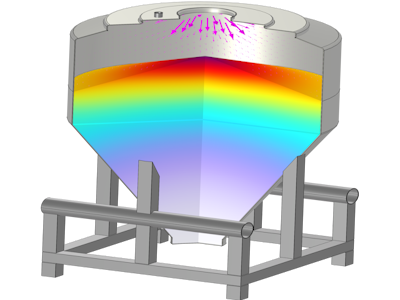
COMSOL Multiphysics® was utilized to develop a robust multiphysics model simulating water vapor diffusion and sorption within pharmaceutical materials. The model incorporates material-specific parameters such as moisture sorption isotherms, diffusion coefficients, particle size, compressibility, and permeability of pharmaceutical excipients. The problem was defined by integrating multiple physics modules including Transport of Diluted Species interface, Heat Transfer and Porous Media and Subsurface Flow modules.
Experimental validation was conducted using custom-designed setups that tracked moisture migration behavior in real-time within controlled environments. Moisture-sensitive pharmaceutical powder beds were exposed to controlled headspace relative humidity (RH), with continuous in-situ monitoring using RH sensors. The temporal evolution of RH within the bed and, indirectly, the moisture uptake by the solid sample, provided direct experimental data for comparison and validation of the COMSOL predictions.
Our results demonstrate strong agreement between the COMSOL model's predictions for moisture ingress rates and internal moisture distribution and the experimental data obtained from in-situ RH sensor measurements. This integrated modeling and experimental approach not only deepens the understanding of moisture transport mechanisms in various pharmaceutical matrices but also facilitates the identification of critical moisture hotspots and vulnerable processing stages (e.g., open-air transfer, intermediate holding). The developed and validated models can serve as a powerful tool for optimizing storage conditions, guiding the design of improved moisture-barrier packaging, and informing advanced process control strategies to minimize moisture-induced degradation, thereby enhancing the handleability, robustness, and longevity of pharmaceutical excipients and finished products.