La Galleria delle Applicazioni raccoglie un'ampia varietà di tutorial e di app dimostrative realizzati con COMSOL Multiphysics in diversi ambiti applicativi, inclusi quelli elettrico, meccanico, fluidico e chimico. E' possibile scaricare i file dei modelli e delle app demo pronti all'uso e le istruzioni step-by-step per costruirli, e utilizzarli come punto di partenza per le proprie simulazioni.
Lo strumento di Ricerca Rapida permette di trovare i modelli che si riferiscono alla propria area di interesse.
Si noti che molti degli esempi qui presentati sono accessibili anche tramite le Librerie delle Applicazioni incorporate nel software COMSOL Multiphysics® e disponibili dal menu File.
This tutorial demonstrates how to modify a concentration-independent (also known as a secondary current distribution) liquid alkaline water electrolyzer with concentrated electrolyte theory to explicitly resolve local electrolyte and solvent concentrations (thereby creating a tertiary ... Per saperne di più
This model defines a zero-gap alkaline water electrolyzer, where oxygen and hydrogen gas are evolved in porous gas diffusion nickel felt electrodes, placed adjacent to a porous separator (diaphragm). The geometry defines a unit cell of an electrolyzer stack, in turn comprising two full ... Per saperne di più
In an alkaline electrolyzer stack, all cells share the same electrolyte. As a result of all cells being in ionic contact, parasitic shunt currents flow between the cells through the manifolds and the electrolyte channels, on both the inlet and outlet side. This example models a ... Per saperne di più
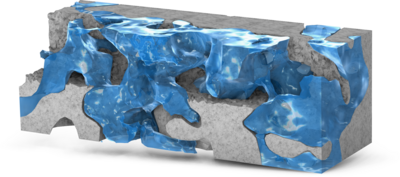
Digital Rock technology utilizes high-resolution imaging techniques, such as SEM and X-ray CT, to characterize the pore structures of rock cores. This approach enables pore-scale modeling of reservoir rocks, offering valuable insights into pore morphology, connectivity, and fluid ... Per saperne di più
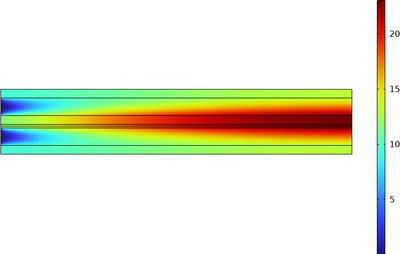
The chlor-alkali membrane process is one of the largest in industrial electrolysis with the production of roughly 40 million metric tons of both chlorine and caustic soda per year. Chlorine is used predominantly for the production of vinyl chloride monomer, which in turn is used for the ... Per saperne di più
This example models co-electrolysis of H2O and CO2 using a solid oxide electrolyzer cell. The model includes the full coupling between the mass balances and gas flow in the H2 and O2 gas diffusion electrodes, the momentum balances in the H2 and O2 gas flow channels, the energy balance ... Per saperne di più
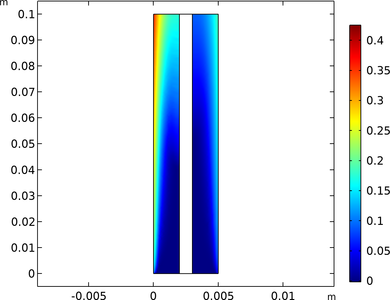
Alkaline water electrolysis is a well-established industrial process for producing hydrogen gas. In the cell, hydrogen gas is formed at the cathode whereas oxygen gas is formed at the anode. The electrolyte is an aqueous liquid, and when the evolved gases form bubbles, the effective ... Per saperne di più
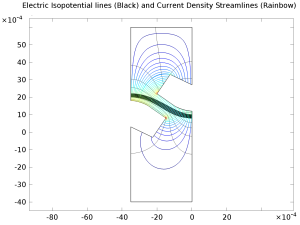
This model file was used for creating the plots featured in the blog post "How to Model Ion-Exchange Membranes and Donnan Potentials". Per saperne di più
A bipolar membrane consists of one anion-selective, and one cation-selective membrane, in contact with each other. The combined cation and anion selectivity makes the bipolar membrane highly impermeable to all ions, with the exception of H+ and OH- which are formed by water splitting ... Per saperne di più
Metal hydride tanks offer safe hydrogen storage, thanks to their low reactivity, and a relatively high hydrogen density. When developing metal hydride hydrogen tank designs, modeling and simulation is useful for optimizing operating conditions, such as gas composition, pressure, and ... Per saperne di più